Background: Flow Cytometry is the current standard for detection of measurable residual disease (MRD) in T-lymphoblastic leukemia (T-ALL) for North America but relies on subjective interpretation of data by a highly trained interpreter, which raises concerns for reproducibility between laboratories. Nevertheless, MRD > 0.01% at End of Induction (EOI) and End of Consolidation (EOC) have been associated with poorer outcome in T-ALL. MRD detection by next-generation sequencing (NGS) offers the potential for reduced subjectivity and increased sensitivity, as has already been demonstrated in B-lymphoblastic leukemia. This study assesses the performance characteristics and potential contribution of NGS for the detection of T-ALL MRD in pediatric patients.
Method: Residual patient samples from COG AALL1231 concurrently tested for T-ALL MRD by flow cytometry were tested for the presence of leukemia-associated clonal TCRB sequences at the University of Washington using the immunoSEQ kit from Adaptive Biotechnologies. The sensitivity of the assay for these samples is estimated at 0.002%. The samples included 615 pretreatment samples, 298 EOI samples, and 72 EOC samples. The EOI samples were negative for MRD by flow cytometry (MRD<0.01%). The EOC samples were from patients positive for MRD at EOI, as per the treatment protocol. 111 samples lacking a pretreatment clonal TCRB sequence for which residual material was available were submitted to Adaptive Biotechnologies for TCRG sequencing. ETP status was determined on pre-treatment samples by flow cytometry. A pretreatment clonal sequence representing > 19% of the total T cell sequences was considered leukemia-associated and the presence any leukemia-associated clonal sequence post-treatment was considered MRD positive. The MRD results were correlated with clinical outcome.
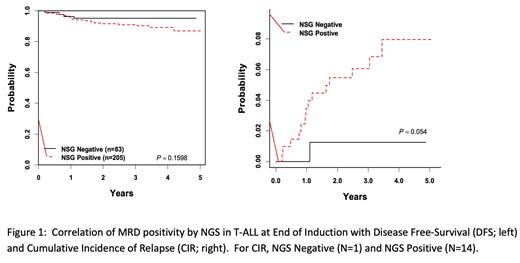
Results: Clonal TCRB leukemia-associated sequences were identified in 68.5% of pretreatment samples and correlated with ETP status (ETP 3.6% (N=56) , Near-ETP 44.8% (N=96), Not-ETP 82.4% (N=449), unknown 50% (N=14)). Clonal TCRG leukemia-associated sequences were identified in an additional 44.1% of pretreatment samples lacking a clonal TCRB sequence (N=111). EOI MRD was identified in 71.0% of samples assayed for TCRB (ETP not evaluable (N=0), Near-ETP 76.2% (N=21), Not-ETP 70.3% (N=263), Unknown 77.8% (N=9)). Additional EOI samples not informative for TCRB and assayed for TCRG (N=29) identified 89.7% as MRD positive. EOC MRD was identified in 63.9% of samples assayed for TCRB (ETP 66.7% (N=3), Near-ETP 45.5% (N=11), Not-ETP 68.4% (N=57), Unknown 0% (N=1)). Additional EOC samples not informative for TCRB and assayed for TCRG (N=35) identified as 68.6% MRD positive. Correlation of MRD status with clinical outcome data revealed no difference in disease-free survival (DFS) at either EOI or EOC, although all relapses but one at each timepoint (N=15 EOI, N=15 EOC) occurred in the MRD positive groups.
Conclusion: A significant subset of T-ALL samples do not contain clonal leukemia-associated TCRB or TCRG sequences (31.5% for TCRB, 17.6% using both TCRB or TCRG) and cannot be evaluated for MRD using this NGS approach. Samples lacking a clonal leukemia-associated sequence are associated with an immature immunophenotype (ETP or Near-ETP), although 17.6% of Not-ETP samples also lack a clonal sequence, suggesting that flow cytometric classification of maturational stage is likely imperfect. A high frequency of MRD positivity is present at both EOI and EOC that does not reflect the excellent outcome that most patients experience, thus raising concerns about the specificity of these clonal sequences at very low levels of detection. Evaluation of sequence specificity across the cohort and use of higher MRD thresholds to optimize the correlation with clinical outcome is underway. A negative MRD result by NGS has a high negative predictive value for relapse/death at either EOI (95.2%) or EOC (92.9%) but a poor positive predictive value (6.8% at both EOI and EOC).
Disclosures
Teachey:Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; BEAM: Research Funding; Neoimmune Tech: Research Funding. Raetz:Pfizer: Research Funding; Bristol Myer Squibb: Other: DSMC. Hunger:Servier: Honoraria; Novartis: Consultancy; Jazz: Honoraria; Amgen: Current equity holder in publicly-traded company, Honoraria. Wood:Beckman-Coulter: Honoraria; Wugen: Other: Institutional Laboratory Services Agreement; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional Laboratory Services Agreement; Becton-Dickinson: Honoraria; Kite: Other: Institutional Laboratory Services Agreement; Novartis: Other: Institutional Laboratory Services Agreement; Macrogenics: Other: Institutional Laboratory Services Agreement; Beam: Other: Institutional Laboratory Services Agreement; Biosight: Other: Institutional Laboratory Services Agreement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal